Bulbar function
Components of bulbar function in patients with SMA
Descriptive post hoc analyses of ZOLGENSMA clinical trial data
SMA experts and Novartis Gene Therapies have published post hoc analyses of data from ZOLGENSMA clinical trials to evaluate bulbar function in patients who were presymptomatic and symptomatic for SMA at the time of ZOLGENSMA infusion. Both analyses were descriptive only, no hypotheses were considered. Categorical variables are described as numbers and percentages.
Post hoc analyses of data from completed clinical trials were conducted. All trials included patients with biallelic mutations in the SMN1 gene.1,2
Presymptomatic study
SPR1NT (N=29) was an open-label, single-arm, Phase 3, clinical trial3:
The primary endpoints where functional, independent sitting for ≥30 seconds (Bayley item 26) up to 18 months of age (2 copies) and ability to stand without support for ≥3 seconds (Bayley item 40) up to 24 months of age (3 copies).
The secondary endpoints event-free survival at 14 months of age (2 copies),a ability to maintain weight at or above the 3rd percentile, based on WHO Child Growth Standards, without nutritional support up to 18 months of age (2 copies), and ability to walk alone (Bayleyb) up to 24 months of age (3 copies).
BiPAP=Bilevel Positive Airway Pressure; CHOP INTEND=Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders; WHO=World Health Organization.
aEvent-free survival was defined as avoidance of both death and permanent ventilation (tracheostomy or ≥16 hours respiratory assistance per day continuously for ≥14 days in the absence of an acute reversible illness, excluding perioperative ventilation).
bBayley-III, gross motor subtest item 43, defined by the ability to take ≥5 steps independently displaying coordination and balance.
Symptomatic studies:
START (N=15) was an open-label, single-arm, Phase 1, dose-escalation clinical trial4:
The primary endpoint was 24-month safety. The secondary endpoints were event-free survivala and change from baseline in CHOP INTEND scores.
STR1VE-EU (N=33) was an open-label, single-arm, Phase 3 clinical trial5:
The primary endpoint was independent sitting for at least 10 seconds by the 18-months-of-age study visit (WHO). The secondary endpoint was event-free survival at 14-months-of-age study visit.a
STR1VE-US (N=22) was an open-label, single-arm, Phase 3 clinical trial6:
Primary endpoints were event-free survival at 14-months-of-age study visita and functional, independent sitting for ≥30 seconds at 18-months-of-age study visit. The secondary endpoints were ability to thrive at 18 months of age, a composite endpoint and independence from ventilatory support at 18 months of age (based solely on Trilogy BiPAP data).b
aEvent is defined as death or the need for permanent ventilatory support consisting of ≥16 hours of respiratory assistance per day continuously for ≥14 days in the absence of an acute reversible illness, excluding perioperative ventilation.
bMaintains weight (≥3rd percentile for age and gender, based on WHO Child Growth Standards), does not receive nutrition through mechanical support, and able to tolerate thin liquids as demonstrated through a formal swallowing test.
Methods1,2
In these analyses, bulbar function was defined as: Integrity within cranial nerves that enables an individual to (1) meet nutritional needs by mouth without pulmonary instability and (2) demonstrate verbal communication abilities.
Four endpoints from available clinical trial data were selected to represent key components of bulbar function.
Together these components formed the composite endpoint of bulbar function. Verbal communication was not assessed in SPR1NT and not included in the composite analysis for presymptomatic patients.2
Airway protection
No occurrence of adverse events (such as aspiration or aspiration pneumonia) due to compromised airway protection.
Swallowing
No evidence of swallowing impairment identified by a clinician (via clinical or fluoroscopic markers).
Full oral nutrition
Received 76-100% of nutrition orally (per HCP or parent report) and did not require non-oral feeding support.
Verbal communication
Vocalize at least 2 different, distinct vowel sounds (achievement of item 6 or above on the Bayley Scales of Infant and Toddler Development, 3rd Edition Expressive Communication subtest).
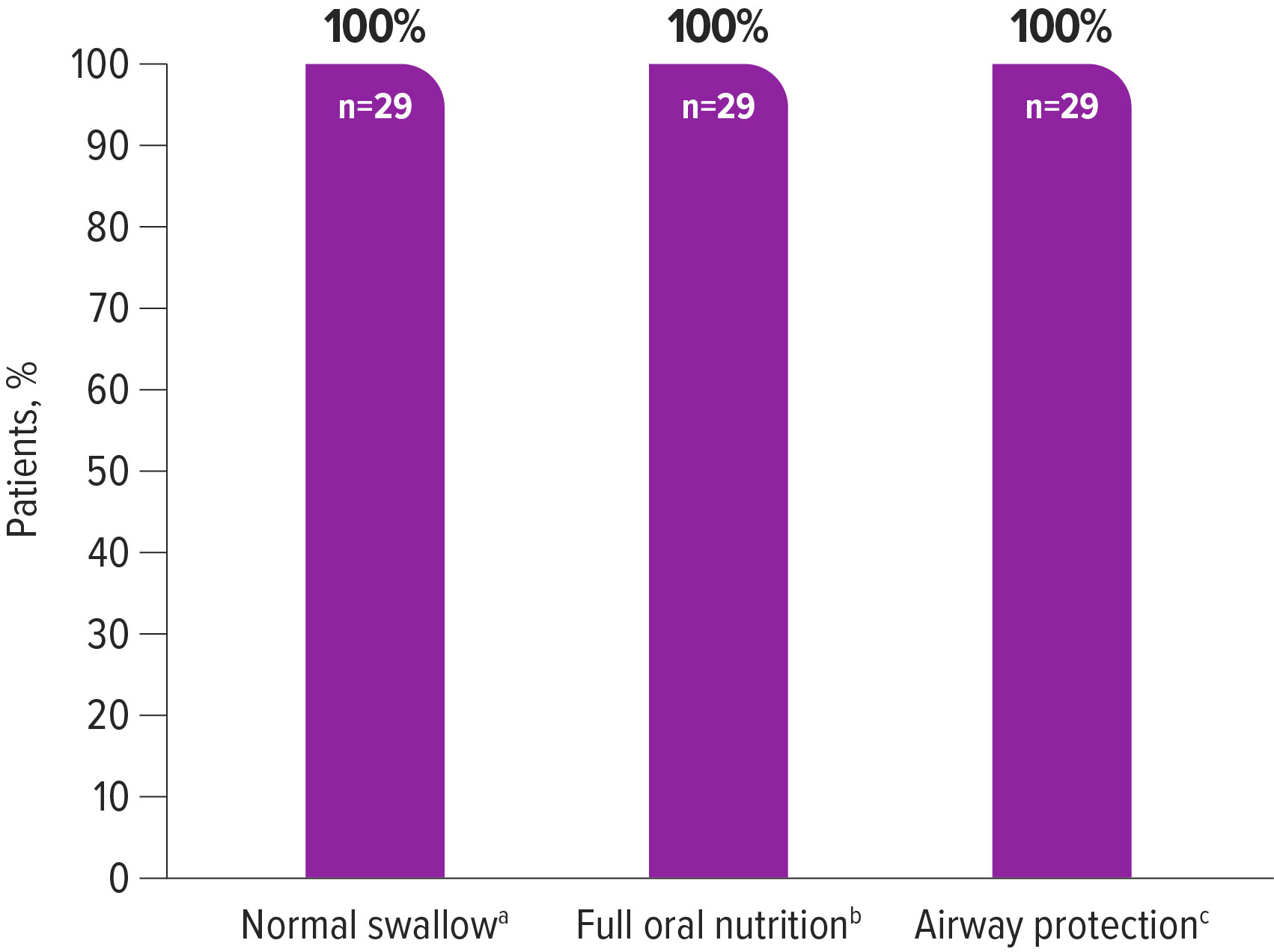
Descriptive post hoc analysis of data from 29 presymptomatic patients treated with ZOLGENSMA1
In the post hoc analysis, data for normal swallow, full oral nutrition, and airway protection from the SPR1NT trial were reviewed. Verbal communication was not measured in SPR1NT and was therefore not included in the analysis. Performance at the last evaluated time point was used to judge achievement of individual and composite bulbar function outcomes.
100% (29/29) of patients achieved the available individual and composite endpoints for bulbar function after ZOLGENSMA treatment
aSwallowing was assessed by a clinical bedside examination or a fluoroscopic examination according to standard practice and clinician decision at each study site.
bThe need for nutrition support and the percentage of nutrition received via oral intake were assessed via parent report or provider observation at the end of study visit. A lack of non-oral nutrition support (i.e., enteral nutrition via feeding tube or parenteral nutrition) and receiving 76–100% of nutrition via oral intake together represented maximum oral nutrition for this analysis.
cA lack of adverse events of aspiration or aspiration pneumonia was considered evidence of the ability to maintain pulmonary stability.
aSwallowing was assessed by a clinical bedside examination or a fluoroscopic examination according to standard practice and clinician decision at each study site.
bThe need for nutrition support and the percentage of nutrition received via oral intake were assessed via parent report or provider observation at the end of study visit. A lack of non-oral nutrition support (i.e., enteral nutrition via feeding tube or parenteral nutrition) and receiving 76–100% of nutrition via oral intake together represented maximum oral nutrition for this analysis.
cA lack of adverse events of aspiration or aspiration pneumonia was considered evidence of the ability to maintain pulmonary stability.
Descriptive post hoc analysis of pooled data from 65 symptomatic patients treated with ZOLGENSMA2
This analysis included pooled data from START, STR1VE-US, and STR1VE-EU.
Data for normal swallow, full oral nutrition, airway protection and verbal communication were reviewed.a-d
Patients were included in this post hoc analysis if they were <6 months of age at the time of infusion and received the therapeutic dose (or equivalent).
Performance at the last evaluated time point was used to judge achievement of individual and composite bulbar function outcomes.
75% (15/20) of patients with data on all 4 endpoints as of the last evaluated time point, met the composite endpoint for bulbar functione,f
aSwallowing was assessed by a clinical bedside examination or a fluoroscopic examination according to standard practice and clinician decision at each study site.
bThe need for nutrition support and percentage of oral intake was assessed via parent report or provider observation. A lack of non-oral nutrition support and receiving 76–100% of nutrition via oral intake together represented maximum oral nutrition for this analysis.
cA lack of adverse events of aspiration or aspiration pneumonia was considered evidence of the ability to maintain pulmonary stability.
dCommunication was only assessed for patients from native English-speaking families in START and STR1VE-US. The communication endpoint for this post-hoc analysis was considered achievement of item #6 or greater on the Bayley Expressive Communication subtest.
ePercentages calculated as a proportion of patients with available data (N=65 for swallow, nutrition, and airway protection; N=20 for communication). End of study represents the last evaluated timepoint during the study period.
fCommunication was only assessed for patients from native English-speaking families in START and STR1VE-US. At baseline, communication was assessed for 20 patients in STR1VE-US; before end of study, communication was assessed for 4 patients in START and 16 in STR1VE-US.
Individual endpoints for bulbar functione,f
| Normal swallow n (%) | Full oral nutrition n (%) | Airway protection n (%) | Verbal communication n (%) | |
|---|---|---|---|---|
| START (N=11) | ||||
| Baseline End of study | 4 (36) 11 (100) | 7 (64) 6 (55) | 11 (100) 8 (73) | – 4 (100) |
| STR1VE-US (N=22) | ||||
| Baseline End of study | 22 (100) 20 (91) | 22 (100) 19 (86) | 22 (100) 20 (91) | 4 (20) 15 (94) |
| STR1VE-EU (N=32) | ||||
| Baseline End of study | 31 (97) 29 (91) | 26 (81) 24 (75) | 32 (100) 32 (100) | – – |
| Combined (N=65) | ||||
| Baseline End of study | 57 (88) 60 (92) | 55 (85) 49 (75) | 65 (100) 60 (92) | 4 (20) 19 (95) |
aSwallowing was assessed by a clinical bedside examination or a fluoroscopic examination according to standard practice and clinician decision at each study site.
bThe need for nutrition support and percentage of oral intake was assessed via parent report or provider observation. A lack of non-oral nutrition support and receiving 76–100% of nutrition via oral intake together represented maximum oral nutrition for this analysis.
cA lack of adverse events of aspiration or aspiration pneumonia was considered evidence of the ability to maintain pulmonary stability.
dCommunication was only assessed for patients from native English-speaking families in START and STR1VE-US. The communication endpoint for this post-hoc analysis was considered achievement of item #6 or greater on the Bayley Expressive Communication subtest.
ePercentages calculated as a proportion of patients with available data (N=65 for swallow, nutrition, and airway protection; N=20 for communication). End of study represents the last evaluated timepoint during the study period.
fCommunication was only assessed for patients from native English-speaking families in START and STR1VE-US. At baseline, communication was assessed for 20 patients in STR1VE-US; before end of study, communication was assessed for 4 patients in START and 16 in STR1VE-US.
References: 1. Shell RD, McGrattan KE, Hurst-Davis R, et al. Onasemnogene abeparvovec preserves bulbar function in infants with presymptomatic spinal muscular atrophy: a post-hoc analysis of the SPR1NT trial. Neuromuscul Disord. 2023;33(8):670-676. 2. McGrattan KE, Shell RD, Hurst-Davis R, et al. Patients with spinal muscular atrophy type 1 achieve and maintain bulbar function following onasemnogene abeparvovec treatment. J Neuromuscul Dis. 2023;10(4):531- 540. 3. Novartis Gene Therapies, Inc. Pre-symptomatic study of intravenous onasemnogene abeparvovec-xioi in spinal muscular atrophy (SMA) for patients with multiple copies of SMN2 (SPR1NT). https://www.clinicaltrials.gov/study/NCT03505099. ClinicalTrials.gov identifier: NCT03505099. Updated September 7, 2022. Accessed October 19, 2023. 4. Novartis Gene Therapies, Inc. Gene transfer clinical trial for spinal muscular atrophy type 1. https://www.clinicaltrials.gov/study/NCT02122952. ClinicalTrials.gov identifier: NCT02122952. Updated September 15, 2022. Accessed October 19, 2023. 5. Novartis Gene Therapies, Inc. Single-dose gene replacement therapy clinical trial for participants with spinal muscular atrophy Type 1 (STRIVE-EU). https://www.clinicaltrials.gov/study/NCT03461289. Updated August 16, 2022. Accessed October 19, 2023. 6. Novartis Gene Therapies, Inc. Gene replacement therapy clinical trial for participants with spinal muscular atrophy type 1 (STR1VE). https://www.clinicaltrials.gov/study/NCT03306277. ClinicalTrials.gov identifier: NCT03306277. Updated August 16, 2022. Accessed October 19, 2023.