Steps to treatment: After
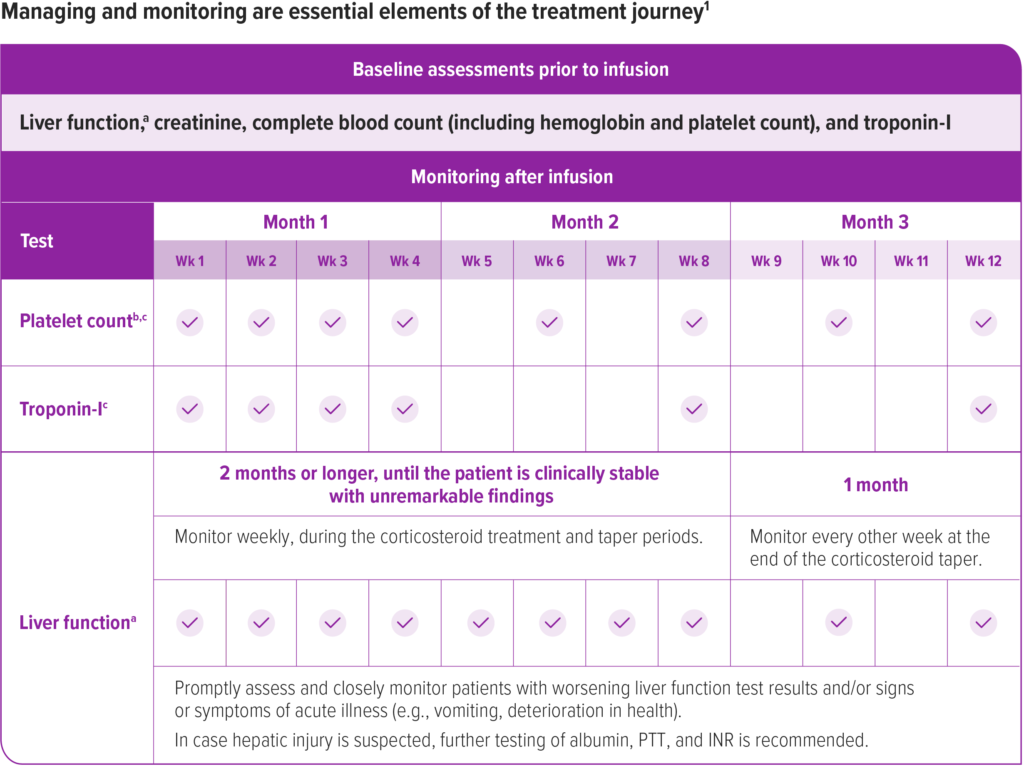
Management and monitoring are essential elements of the treatment journey
Prior to infusion, ensure patients have baseline tests for liver function,a creatinine, complete blood count (including hemoglobin and platelet count), and troponin-I.1
After infusion, continue to monitor liver function, platelet count,b and troponin-I as described in the Prescribing Information. See the recommended schedule of assessments in the accompanying chart.1
Due to the increased risk of serious systemic immune response, administer ZOLGENSMA to patients who are clinically stable in their overall baseline health status (e.g., hydration and nutritional status, absence of infection) prior to infusion. Postpone ZOLGENSMA in patients with infections until the infection has resolved and the patient is clinically stable. Clinical signs or symptoms of infection should not be evident at the time of ZOLGENSMA infusion.
Wk=week.
aLiver function assessment includes a clinical exam and laboratory testing of hepatic aminotransferases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]), total bilirubin, albumin, prothrombin time, partial thromboplastin time (PTT), and international normalized ratio (INR). Monitor liver function for 3 months or longer until results are unremarkable (normal clinical exam, total bilirubin, prothrombin, and INR results, and ALT and AST levels below 2 × ULN).
bMonitor platelet counts as well as signs and symptoms of TMA, such as hypertension, increased bruising, seizures, or decreased urine output.
cMonitor platelet counts and troponin-I levels for 3 months or longer until they return to baseline.
![]() =monitoring performed
=monitoring performed
aLiver function assessment includes a clinical exam and laboratory testing of hepatic aminotransferases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]), total bilirubin, albumin, prothrombin time, partial thromboplastin time (PTT), and international normalized ratio (INR). Monitor liver function for 3 months or longer until results are unremarkable (normal clinical exam, total bilirubin, prothrombin, and INR results, and ALT and AST levels below 2 × ULN).
bMonitor platelet counts as well as signs and symptoms of TMA, such as hypertension, increased bruising, seizures, or decreased urine output.
cMonitor platelet counts and troponin-I levels for 3 months or longer until they return to baseline.
Treat with systemic corticosteroids
To help manage a possible increase in liver aminotransferases, treat all patients with systemic corticosteroids before ZOLGENSMA infusion and continue regimen after ZOLGENSMA infusion1:
The recommended corticosteroid treatment regimen is detailed in the Prescribing Information and in the accompanying chart.
If oral corticosteroid therapy is not tolerated, consider intravenous corticosteroids as clinically indicated.
Day 1: 24 hours prior to infusion
Initiate systemic corticosteroid regimen equivalent to oral prednisolone at 1 mg/kg/day.
Day 2: Infusion day
Infuse ZOLGENSMA and continue the corticosteroid regimen.
Day 30: 28 days after infusion
Check liver status clinically and by assessing ALT, AST, total bilirubin, prothrombin time, and INR.
Do not stop systemic corticosteroids abruptly.
If unremarkable (normal clinical exam, total bilirubin, prothrombin time, and INR; ALT and AST levels below 2 x ULN):
- Then taper the corticosteroid dose gradually over the next 28 days
If liver function abnormalities are present:
- Consult a pediatric gastroenterologist or hepatologist
- Continue systemic corticosteroids (equivalent to oral prednisolone at 1 mg/kg/day) until AST and ALT values are both below 2 × ULN and all other assessments return to normal range
- Then taper the corticosteroid dose gradually over the next 28 days or longer if needed
30-day monitor following steroid taper
Once the patient is clinically stable with unremarkable findings at the end of the taper period continue to monitor liver function every other week for another month.
ALT=alanine aminotransferase; AST=aspartate aminotransferase; ULN=upper limit of normal; INR=international normalized ratio.
aLiver function assessment includes a clinical exam and laboratory testing of AST, ALT, total bilirubin, prothrombin time, and INR.
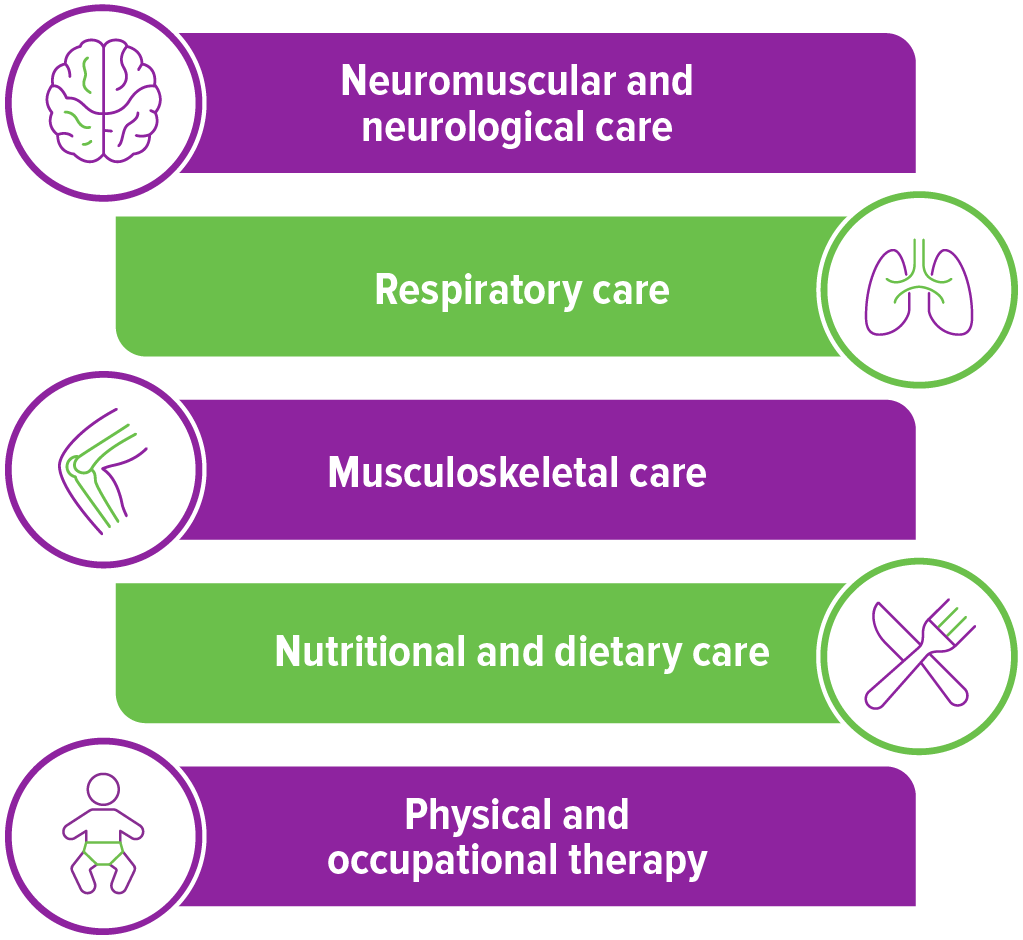
Ongoing care requires a multidisciplinary care team
Even after disease-modifying treatment, patients with SMA may need respiratory, nutritional, and musculoskeletal support. The team will vary from patient to patient, but a coordinated team of specialists can help monitor the patient’s SMA.2-4
It is recommended that a neurological expert, such as a pediatric neurologist or a neuromuscular specialist, coordinate the care of the patient, as they will likely have the most experience with anticipating needs.3,4
While the specific needs and support levels of patients differ, part of the ongoing care can include an assessment of motor function, which may include regular sessions with a physical therapist and possible use of braces. Additionally, nutritionists can be involved to monitor weight and nutrient intake, paying careful attention to calcium and vitamin D for bone health.3
Patient and caregiver support
A resource for caregivers
Help caregivers understand what to expect after treatment and how to maintain proper SMA care. Fill out the Post-Treatment Plan and share it with your patient’s family prior to the patient being discharged.
Post-Treatment Plan
References: 1. ZOLGENSMA [prescribing information]. Bannockburn, IL: Novartis Gene Therapies, Inc; 2023. 2. Finkel RS, Mercuri E, Meyer OH, et al. Diagnosis and management of spinal muscular atrophy: part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018;28(3):197-207. 3. Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103-115. 4. Wang CH, Finkel RS, Bertini ES, et al; Participants of the International Conference on SMA Standard of Care. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22(8):1027-1049.
