Efficacy: SPR1NT trial
SPR1NT: An open-label, single-arm clinical trial of presymptomatic patients with SMA1
SPR1NT was a clinical trial of patients with SMA who have 2 or 3 copies of SMN2 (N=30)1,a
All patients enrolled in the study were less than 6 weeks of age and did not display any symptoms of SMA at the time of infusion1
aOne enrolled patient had 4 copies of SMN2 and is not included in the efficacy analysis. This patient is included in the safety population only.
EXPAND ENDPOINTS AND ENROLLMENT CRITERIA
Endpoints1
2 copies of SMN2 (n=14)
Primary endpoint:
- Functional, independent sitting for ≥30 seconds (Bayley item 26) up to 18 months of age
Secondary endpoints:
- Event-free survival at 14 months of ageb
- Ability to maintain weight at or above the 3rd percentile, based on WHO Child Growth Standards, without nutritional support up to 18 months of age
Exploratory endpoints:
- Other motor milestones (WHO)c
- Gross and fine motor subtests (Bayley)
- Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) scores
3 copies of SMN2 (n=15)
Primary endpoint:
- Ability to stand without support for ≥3 seconds (Bayley item 40) up to 24 months of age
Secondary endpoint:
- Ability to walk alone (Bayleyd) up to 24 months of age
Exploratory endpoints:
- Other motor milestones (WHO)
- Gross and fine motor subtests (Bayley)
bEvent-free survival was defined as avoidance of both death and permanent ventilation (tracheostomy or ≥16 hours respiratory assistance per day continuously for ≥14 days in the absence of an acute reversible illness, excluding perioperative ventilation).
cWHO-Multicenter Growth Study criteria.
dBayley-III, gross motor subtest item 43, defined by the ability to take ≥5 steps independently displaying coordination and balance.
Key inclusion and exclusion criteria1
Key inclusion criteria:
- Bi-allelic SMN1 mutations (deletion or point mutations) and 2 or 3 copies of SMN2 (inclusive of SMN2 gene modifier [c.859G>C])
- ≤6 weeks of age at the time of infusion
- Ability to tolerate thin liquids at screening
- Compound muscle action potential (CMAP) ≥2 mV at baseline
Key exclusion criteria:
- Anti-AAV9 antibody titers >1:50
- Any clinical signs or symptoms that are strongly suggestive of SMA
- Treatment with an investigational or commercial product given for the treatment of SMA
The SPR1NT trial was divided into 2 cohorts based on SMN2 copy number—patients with 2 copies of SMN2 and those with 3 copies of SMN2.

UPDATE: Long-term follow-up data is available for patients from the SPR1NT study
Study results for patients with 2 copies of SMN2 (n=14)
All patients with 2 copies of SMN2 reached 18-months-of-age (study end) and met the secondary endpoint of survival.2,a
aEvent-free survival was defined as avoidance of both death and permanent ventilation (tracheostomy or ≥16 hours respiratory assistance per day continuously for ≥14 days in the absence of an acute reversible illness, excluding perioperative ventilation) at 14 months of age.
Presymptomatic patients with 2 copies of SMN2 achieved age-appropriate motor milestones, after ZOLGENSMA2
100% (14/14) of patients achieved the primary endpoint of sitting without support for ≥30 seconds up to the 18-months-of-age visit2,a
- 79% (11/14) of patients achieved this milestone within an age-appropriate time period2
- Age-appropriate time periods are based on the WHO MGRS of healthy children living under conditions highly unlikely to constrain growth3
Age-appropriate time periods were defined according to the WHO Multicenter Growth Reference Study (MGRS) established windows of achievements for the development of motor milestones.
aBayley-III, gross motor subtest item 26. WHO MGRS established windows of achievement (1%-99%): 3.8-9.2 months for sitting without support.2,3
bIndependent standing ≥10 seconds assessed by WHO MGRS. WHO MGRS established window of achievement (1%-99%): 6.9-16.9 months for standing alone.2,3
cIndependent walking assessed by WHO MGRS. WHO MGRS established windows of achievement (1%-99%): 8.2-17.6 months for walking alone.2,3

Primary endpoint:
Achieved sitting without support for ≥30 seconds (Bayley)2,a
11 of 14 patients achieved sitting without support within an age-appropriate time period
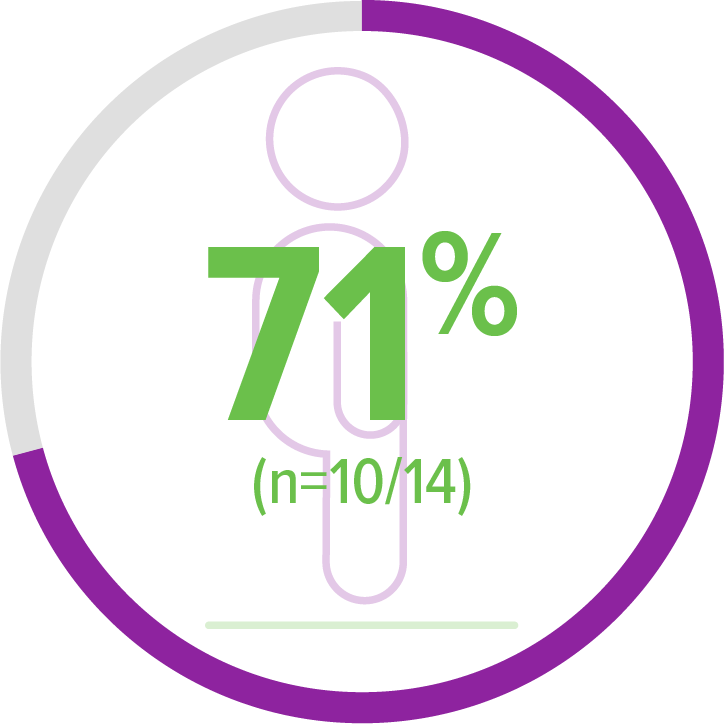
Exploratory endpoint:
Achieved standing alone for ≥10 seconds (WHO)2,b
5 of 10 patients achieved this milestone within an age-appropriate time period
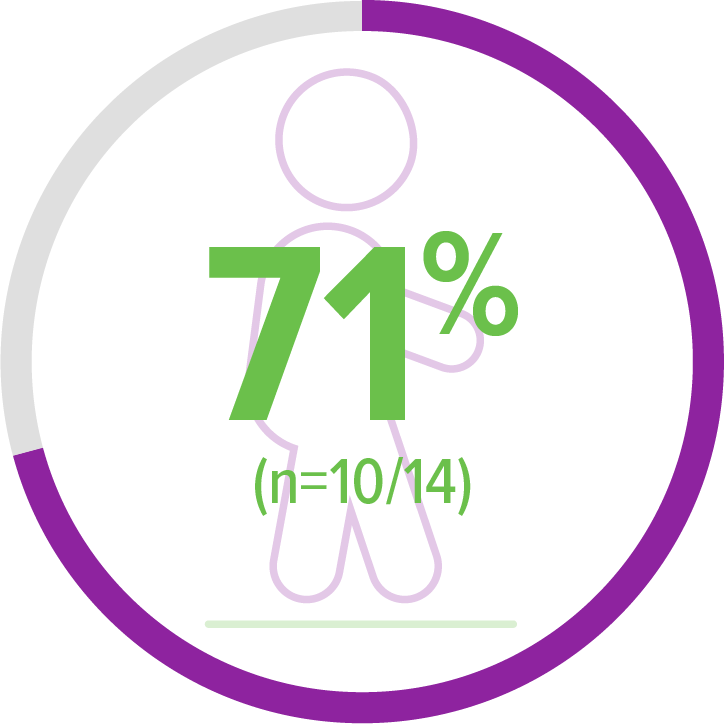
Exploratory endpoint:
Achieved walking alone (WHO)2,c
6 of 10 patients achieved this milestone within an age-appropriate time period
Age-appropriate time periods were defined according to the WHO Multicenter Growth Reference Study (MGRS) established windows of achievements for the development of motor milestones.
Based on participants without health, environmental, or economic constraints on growth.
aBayley-III, gross motor subtest item 26. WHO MGRS established windows of achievement (1%-99%): 3.8-9.2 months for sitting without support.2,3
bIndependent standing ≥10 seconds assessed by WHO MGRS. WHO MGRS established window of achievement (1%-99%): 6.9-16.9 months for standing alone.2,3
cIndependent walking assessed by WHO MGRS. WHO MGRS established windows of achievement (1%-99%): 8.2-17.6 months for walking alone.2,3
ZOLGENSMA enabled continued independence from nutritional support in presymptomatic patients with 2 copies of SMN2 2
aAll patients were required to be able to swallow thin liquids and be free from ventilatory support at baseline.2
Nutritional statusa:
Weight maintenance
(≥3rd percentile for age and gender as defined by WHO)
93% (13/14) of patients
All patients were required to be able to swallow thin liquids and be free from ventilatory support at baseline.2
ZOLGENSMA enabled continued independence from respiratory support in all patients treated presymptomatically2
In natural history, most patients with SMA Type 1 required respiratory support by 12 months of age4
aZOLGENSMA-treated patients in SPR1NT were separated into 2 cohorts by SMN2 copy number. 14 patients had 2 copies of SMN2. 15 patients had 3 copies of SMN2. All patients were required to be able to swallow thin liquids and be free from ventilatory support at baseline. Ventilatory support included noninvasive ventilatory support, invasive ventilatory support, cough assist, or BiPAP.
Respiratory statusa:
Respiratory status
100% (14/14) of patients
aZOLGENSMA-treated patients in SPR1NT were separated into 2 cohorts by SMN2 copy number. 14 patients had 2 copies of SMN2. 15 patients had 3 copies of SMN2. All patients were required to be able to swallow thin liquids and be free from ventilatory support at baseline. Ventilatory support included noninvasive ventilatory support, invasive ventilatory support, cough assist, or BiPAP.
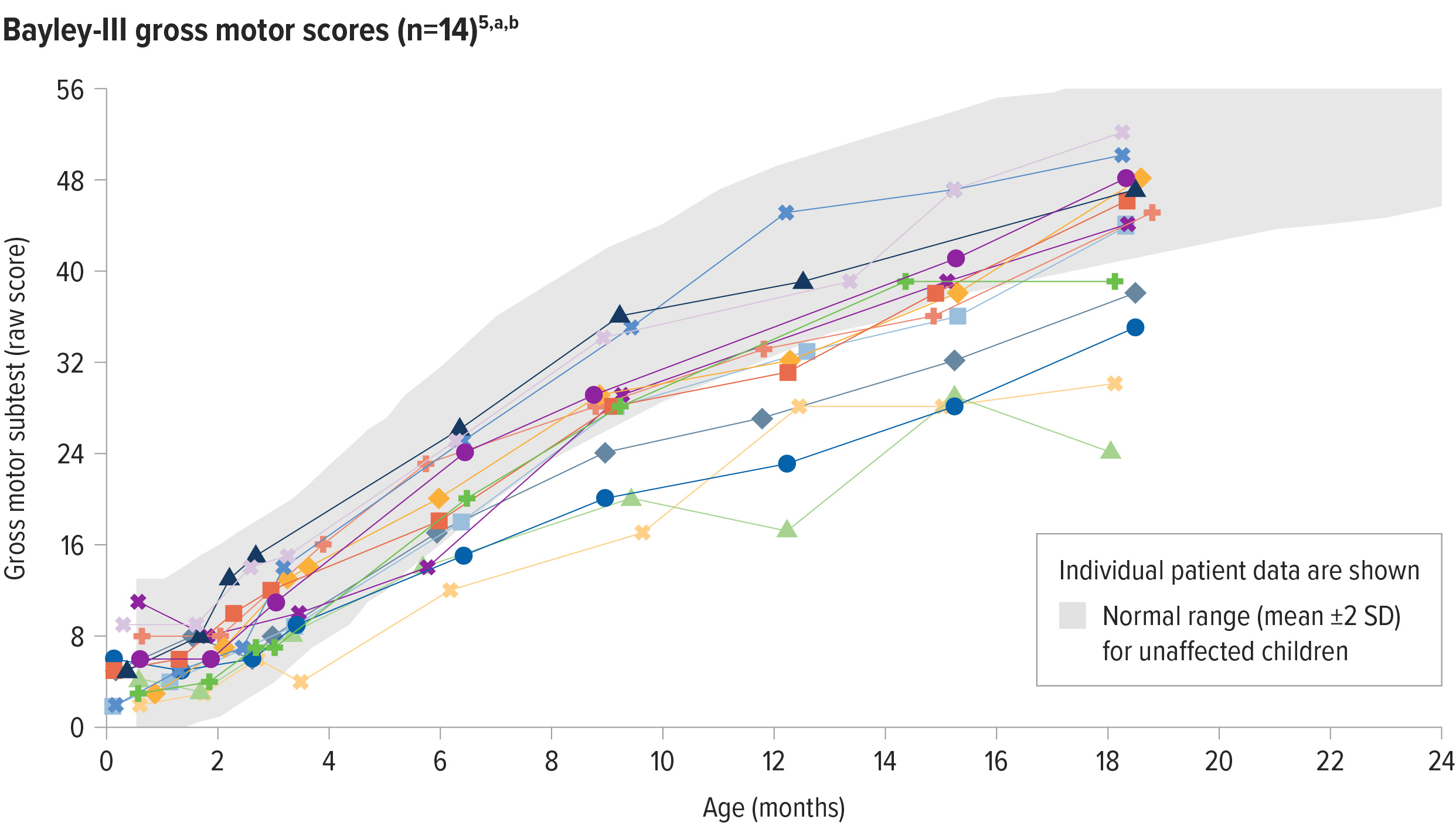
ZOLGENSMA enabled age-appropriate development of gross motor function5
64% (9/14) of patients with 2 copies of SMN2 assessed at study end had gross motor performance similar to same-age children5,a,b
SD=standard deviation.
aGross motor function was measured by the Bayley Scales of Infant and Toddler Development, a well-accepted, standardized tool to assess the development of children between the ages of 1 and 42 months and compared to a standardized norm.6
bEach line represents the raw scores of an individual patient on the Bayley-III gross motor subtest. The normal range for raw scores is based on scaled score equivalents for unaffected patients: mean ± 2 standard deviations (4-16 score [mean = 10, SD = 3, Range of 1-19]).5,6
SD=standard deviation.
aGross motor function was measured by the Bayley Scales of Infant and Toddler Development, a well-accepted, standardized tool to assess the development of children between the ages of 1 and 42 months and compared to a standardized norm.6
bEach line represents the raw scores of an individual patient on the Bayley-III gross motor subtest. The normal range for raw scores is based on scaled score equivalents for unaffected patients: mean ± 2 standard deviations (4-16 score [mean = 10, SD = 3, Range of 1-19]).5,6
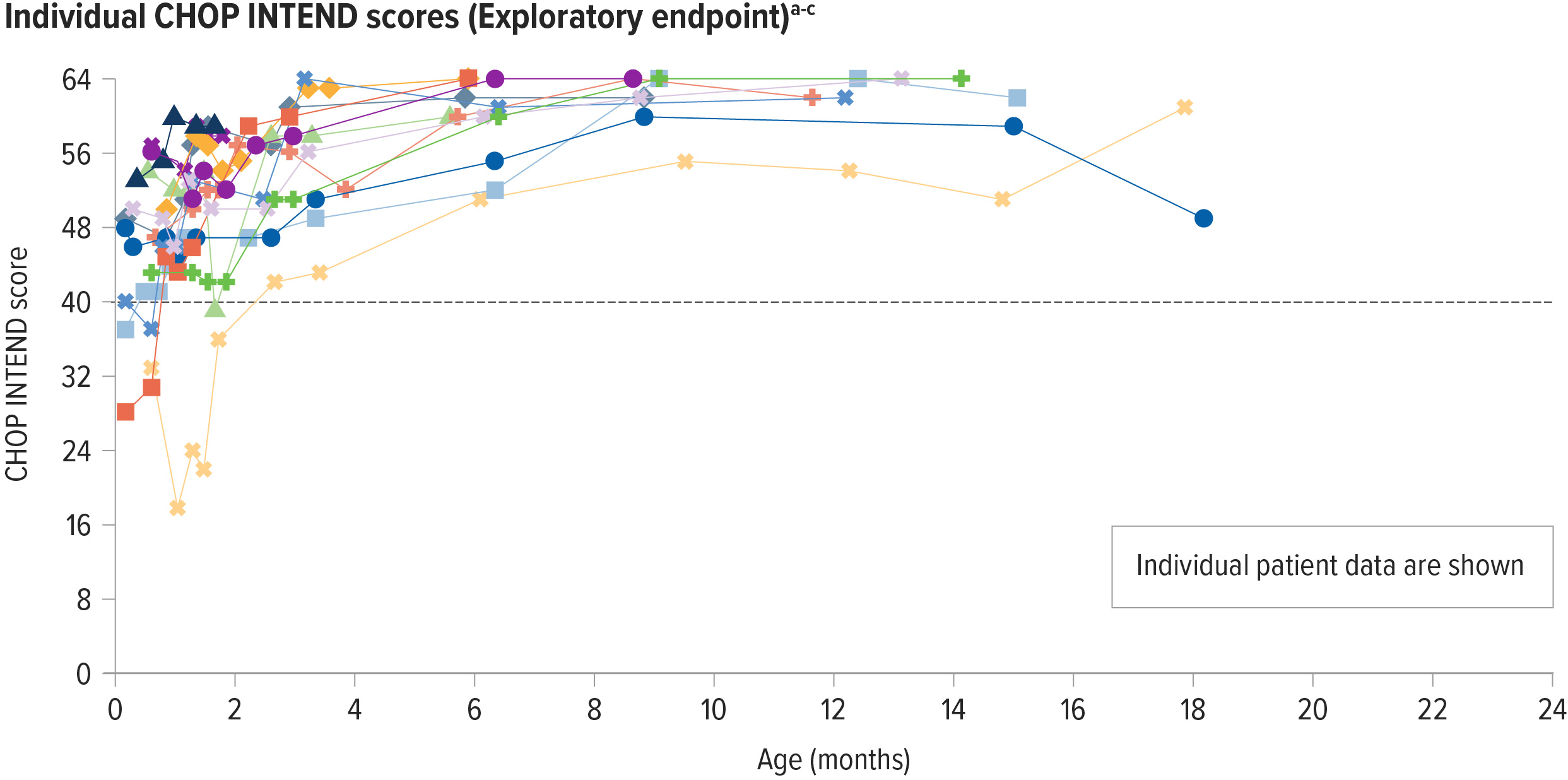
Presymptomatic patients with 2 copies of SMN2 achieved increased motor function scores from baseline
Motor function was assessed using the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND)
CHOP INTEND was specifically developed to assess motor function in patients with SMA. The CHOP INTEND score is measured in points, which are assigned based on a patient’s ability to perform specific motor skills. The test consists of 16 measures, each scored on a scale of 0-4, with the highest possible score being 64 points.7
Increases from baseline in CHOP INTEND were seen as early as 1 month post infusion in patients with 2 copies of SMN2 2
100% (14/14) of patients in the 2-copy cohort achieved a CHOP INTEND score ≥58 points at any visit up to 18 months of age2,a
93% (13/14) of patients achieved a CHOP INTEND score ≥60b
aScores on the CHOP INTEND scale of motor function range from 0 to 64, with higher scores indicating better function.7
bThe patient who did not achieve 60 had achieved 58 or higher 3 times by day 27 post dosing and per protocol did not undergo additional testing.2
cThe dark gray line at 40 represents the highest possible CHOP INTEND score that children with the most severe form of SMA would achieve or maintain, according to natural history.8
SD=standard deviation
aScores on the CHOP INTEND scale of motor function range from 0 to 64, with higher scores indicating better function.7
bOf the 2 remaining patients, 1 patient achieved a maximum post-baseline score of 55 points, and 1 patient achieved 3 consecutive scores ≥58 and, per protocol, did not undergo additional CHOP INTEND examinations.2
cThe dark gray line at 40 represents the highest possible CHOP INTEND score that children with the most severe form of SMA would achieve or maintain, according to natural history.8
Study results for patients with 3 copies of SMN2 (n=15)
All patients with 3 copies of SMN2 reached 24-months-of-age (study end) and were free of permanent ventilation.5,a
aPermanent ventilation (tracheostomy or ≥16 hours respiratory assistance per day continuously for ≥14 days in the absence of an acute reversible illness, excluding perioperative ventilation).5
Presymptomatic patients with 3 copies of SMN2 achieved age-appropriate motor milestones, after ZOLGENSMA5
100% (15/15) of patients achieved the primary endpoint of standing without support up to the 24-months-of-age visit5,a
- 93% (14/15) of patients achieved this milestone within an age-appropriate time period5
- Age-appropriate time periods are based on the WHO MGRS of healthy children living under conditions highly unlikely to constrain growth3
Age-appropriate time periods were defined according to the WHO Multicenter Growth Reference Study (MGRS) established windows of achievements for the development of motor milestones.
Based on healthy children living under conditions highly unlikely to constrain growth. 3
aBayley-III, gross motor subtest item 40. WHO MGRS established windows of achievement (1%-99%): 6.9-16.9 months for standing alone.3,5
bBayley-III, gross motor subtest item 43. WHO MGRS established windows of achievement (1%-99%): 8.2-17.6 months for walking alone.3,5
cStands with assistance as assessed by WHO MGRS. WHO MGRS established windows of achievement (1%-99%): 4.8-11.4 months for standing with assistance.3

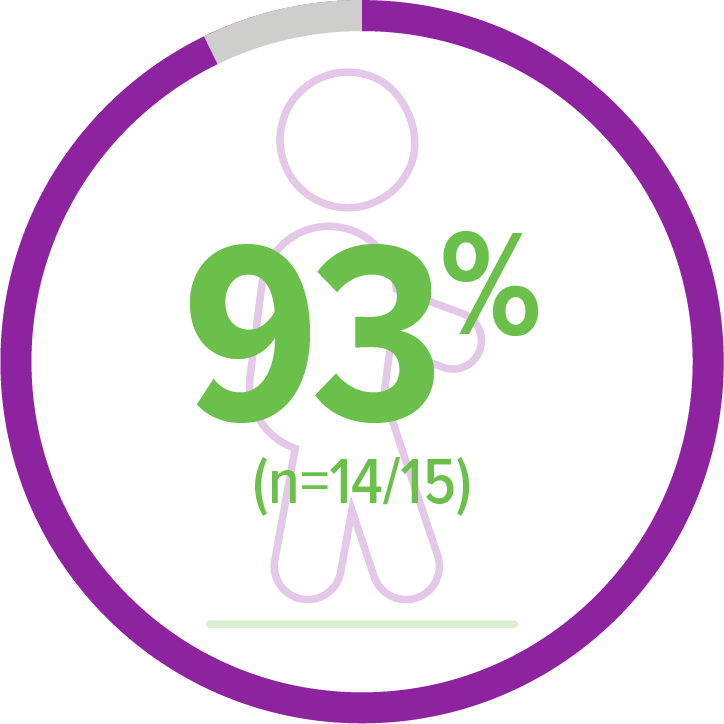
Primary endpoint:
Achieved standing alone for ≥3 seconds (Bayley)5,a
14 of 15 patients achieved this milestone within an age-appropriate time period
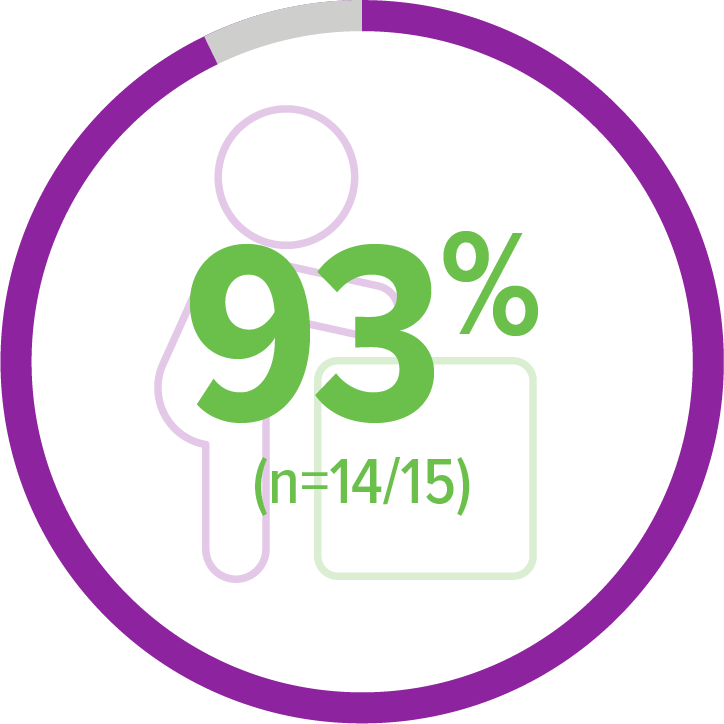
Secondary endpoint:
Achieved walking alone (Bayley)5,b
11 of 14 patients achieved this milestone within an age-appropriate time period
Exploratory endpoint:
Achieved standing with assistance (WHO)5,c
11 of 14 patients achieved this milestone within an age-appropriate time period
Age-appropriate time periods were defined according to the WHO Multicenter Growth Reference Study (MGRS) established windows of achievements for the development of motor milestones.
Based on healthy children living under conditions highly unlikely to constrain growth. 3
aBayley-III, gross motor subtest item 40. WHO MGRS established windows of achievement (1%-99%): 6.9-16.9 months for standing alone.3,5
bBayley-III, gross motor subtest item 43. WHO MGRS established windows of achievement (1%-99%): 8.2-17.6 months for walking alone.3,5
cStands with assistance as assessed by WHO MGRS. WHO MGRS established windows of achievement (1%-99%): 4.8-11.4 months for standing with assistance.3
ZOLGENSMA enabled continued independence from nutritional support in presymptomatic patients with 3 copies of SMN2
aAll patients were required to be able to swallow thin liquids and be free from ventilatory support at baseline.1
Nutritional status5,a:
Feeding support status
100% (15/15) of patients
ZOLGENSMA enabled continued independence from respiratory support in all patients treated presymptomatically5
In natural history, most patients with SMA Type 1 required respiratory support by 12 months of age4
aAll patients were required to be able to swallow thin liquids and be free from ventilatory support at baseline. Ventilatory support included noninvasive ventilatory support, invasive ventilatory support, cough assist, or BiPAP.1
Respiratory statusa:
Respiratory status
100% (15/15) of patients
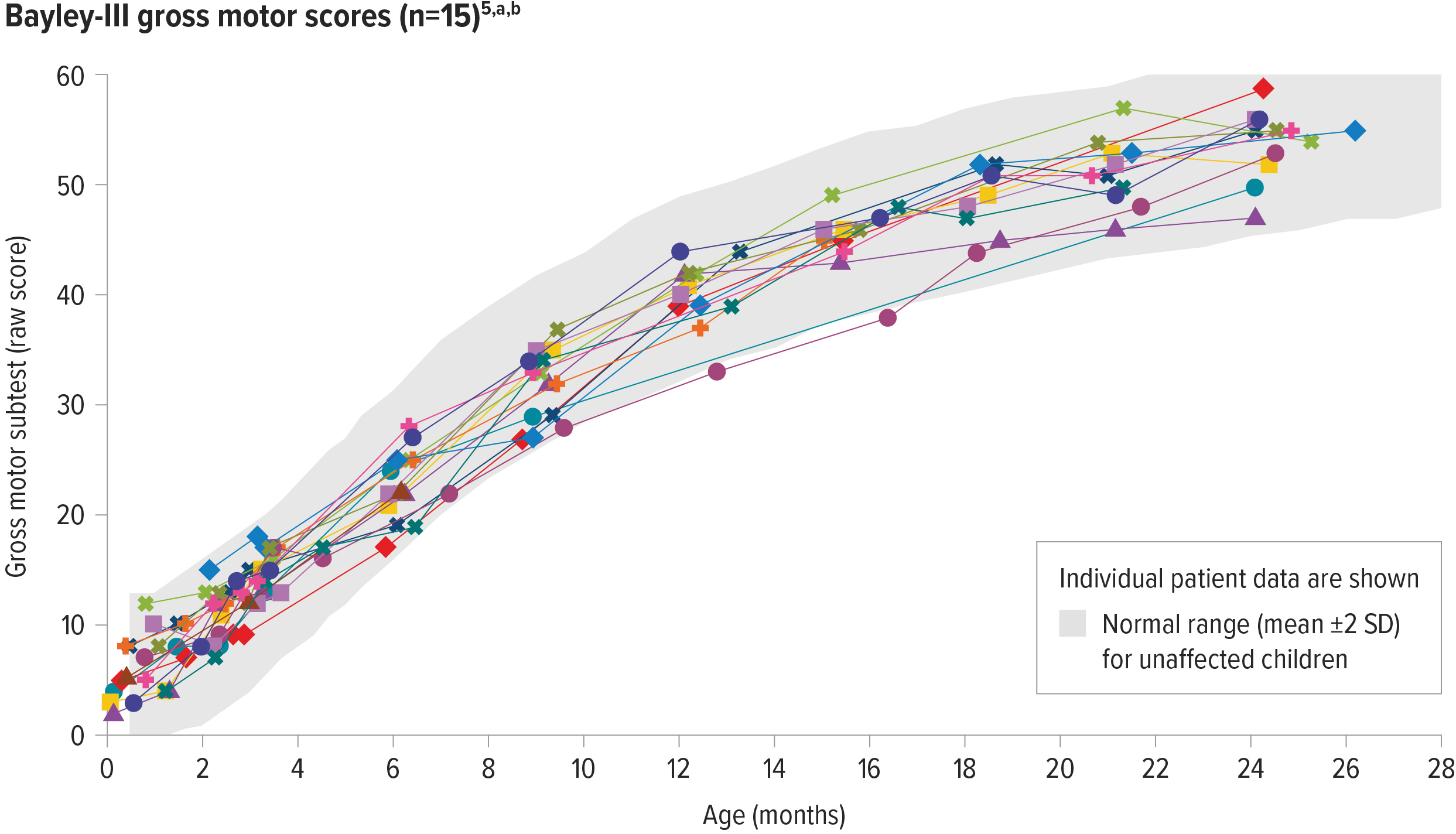
ZOLGENSMA enabled age-appropriate development of gross motor function in patients with 3 copies of SMN2
100% (10/10) of patients assessed had gross motor performance similar to same-aged children at 24 months of age5,a,b
aGross motor function was measured by the Bayley Scales of Infant and Toddler Development, a well-accepted, standardized tool to assess the development of children between the ages of 1 and 42 months, and compared to a standardized norm. The normal range for scores on the gross motor subtest is based on scaled score equivalents for unaffected patients: mean ±2 standard deviations (4-16 score [mean=10, SD=3, range of 1-19]).5,6
bEach line represents the raw scores of an individual patient on the Bayley-III gross motor subtest.
aGross motor function was measured by the Bayley Scales of Infant and Toddler Development, a well-accepted, standardized tool to assess the development of children between the ages of 1 and 42 months, and compared to a standardized norm. The normal range for scores on the gross motor subtest is based on scaled score equivalents for unaffected patients: mean ±2 standard deviations (4-16 score [mean=10, SD=3, range of 1-19]).5,6
bEach line represents the raw scores of an individual patient on the Bayley-III gross motor subtest.
LT-002 is a long-term follow-up study that includes patients from SPR1NT
LT-002 is a 15-year, ongoing, open-label, long-term follow-up of patients who completed a ZOLGENSMA clinical trial. It includes patients with SMA who were either symptomatic or presymptomatic at the time of infusion. 25 patients from the SPR1NT trial have enrolled, including 12 from the 2 copy cohort and 13 from the 3 copy cohort.9,10
The following data are from patients from SPR1NT, who were presymptomatic for SMA at the time of infusion
All patients were alive and none required permanent ventilation in the ongoing trial (as of May 2022)9,a
The mean time since treatment was 3.5 (range 2.9-4.1) years and 3.2 (range 2.8-3.7) years for patients with 2 and 3 copies of SMN2, respectively.9
In long-term follow-up, ZOLGENSMA continues to provide durable efficacy over 4 years post dose.
aThe need for permanent ventilatory support consisting of ≥16 hours of respiratory assistance per day continuously for ≥14 days in the absence of an acute reversible illness or perioperative care.
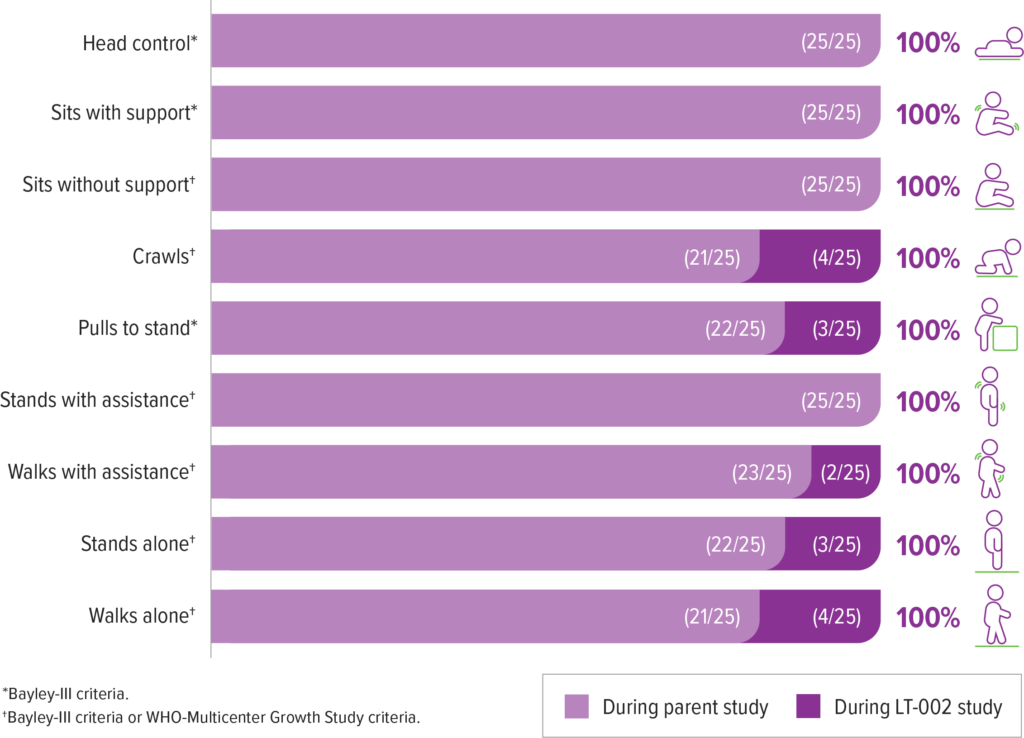
Patients achieved and maintained all motor milestones assessed in the presymptomatic SPR1NT study9
All four patients who did not achieve walking alone in the SPR1NT study achieved this milestone during LT-002 (as of May 2022)9
- 2 of these 4 patients had received other add-on therapy during LT-002 (1 of these 2 patients achieved walking alone before receiving add-on therapy)9,a,b
aAdd-on was defined by treatment with any other disease modifying therapy.9
bIn long-term follow-up some milestone achievements occurred after add-on therapy. Crawls (2/4); Pulls to stand (2/3); Walks with assistance (1/2), Stands alone (1/3), Walks alone (1/4).9
aAdd-on was defined by treatment with any other disease modifying therapy.9
bIn long-term follow-up some milestone achievements occurred after add-on therapy. Crawls (2/4); Pulls to stand (2/3); Walks with assistance (1/2), Stands alone (1/3), Walks alone (1/4).9
References: 1. Novartis Gene Therapies, Inc. Pre-symptomatic study of intravenous onasemnogene abeparvovec-xioi in spinal muscular atrophy (SMA) for patients with multiple copies of SMN2 (SPR1NT). https://clinicaltrials.gov/study/NCT03505099. ClinicalTrials.gov identifier: NCT03505099. Updated September 7, 2022. Accessed October 19, 2023. 2. Data on file. Novartis Gene Therapies, Inc. 2021. 3. WHO Multicentre Growth Reference Study Group. WHO Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatr Suppl. 2006;450:86-95. 4. Finkel RS, McDermott MP, Kaufmann P, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014; 83(9):810-817. 5. Data on file. Novartis Gene Therapies, Inc. 2022. 6. Bayley N. Bayley Scales of Infant and Toddler Development: Administration Manual. 3rd ed. The Psychological Corporation; 2006. 7. Glanzman AM, Mazzone E, Main M, et al. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): test development and reliability. Neuromuscul Disord. 2010;20(3):155-161. 8. Kolb SJ, Coffey CS, Yankey JW, et al; NeuroNEXT Clinical Trial Network on behalf of the NN101 SMA Biomarker Investigators. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82(6):883-891. 9. Data on file. Novartis Gene Therapies, Inc. 2023. 10. Novartis Gene Therapies, Inc. Long-term follow-up study of patients receiving onasemnogene abeparvovec-xioi. https://clinicaltrials.gov/ct2/show/record/NCT04042025. ClinicalTrials.gov identifer: NCT04042025. Updated July 11, 2023. Accessed October 19, 2023.